Experimental and theoretical charge density studies
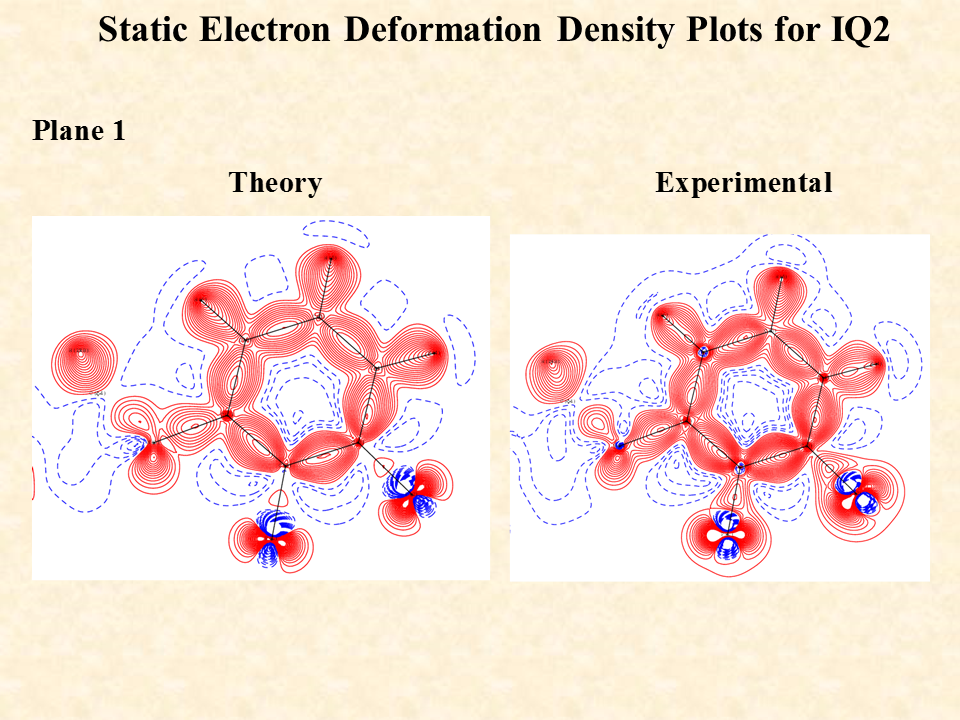
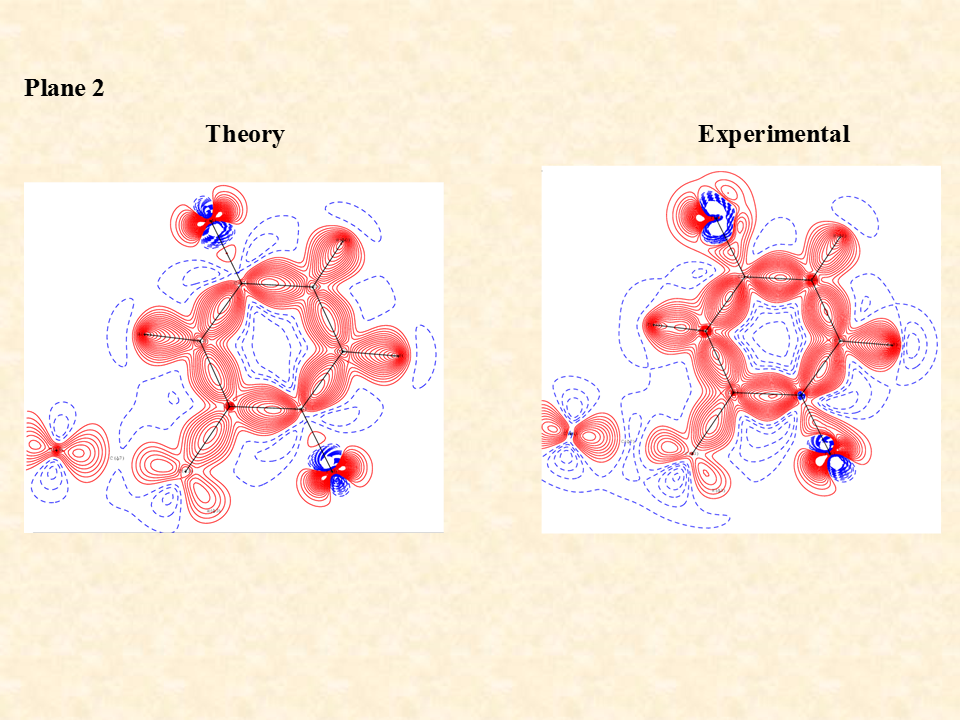
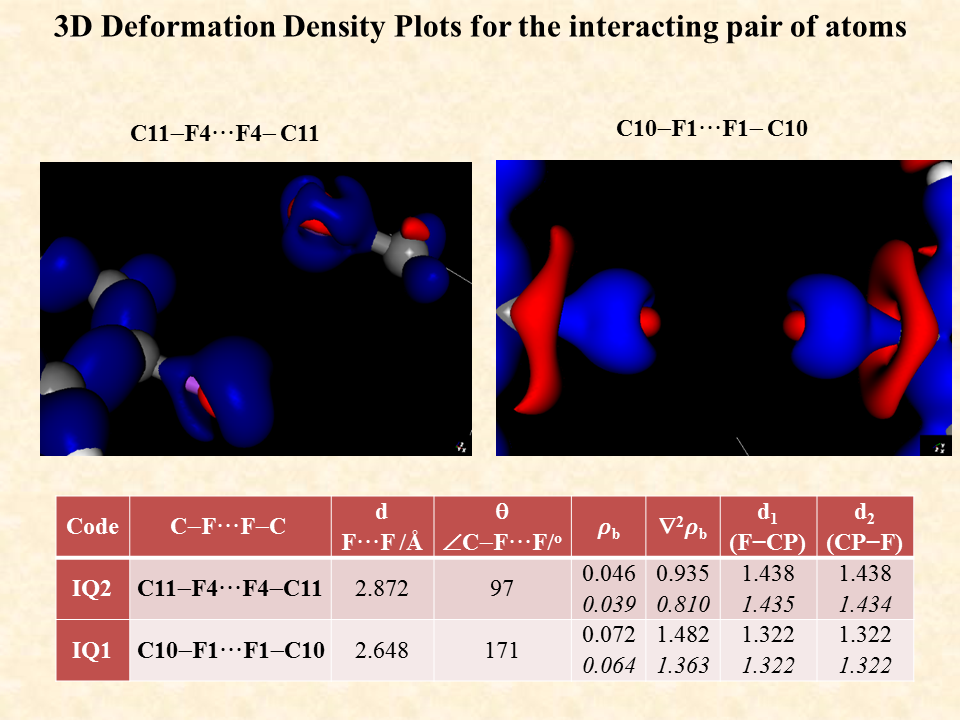
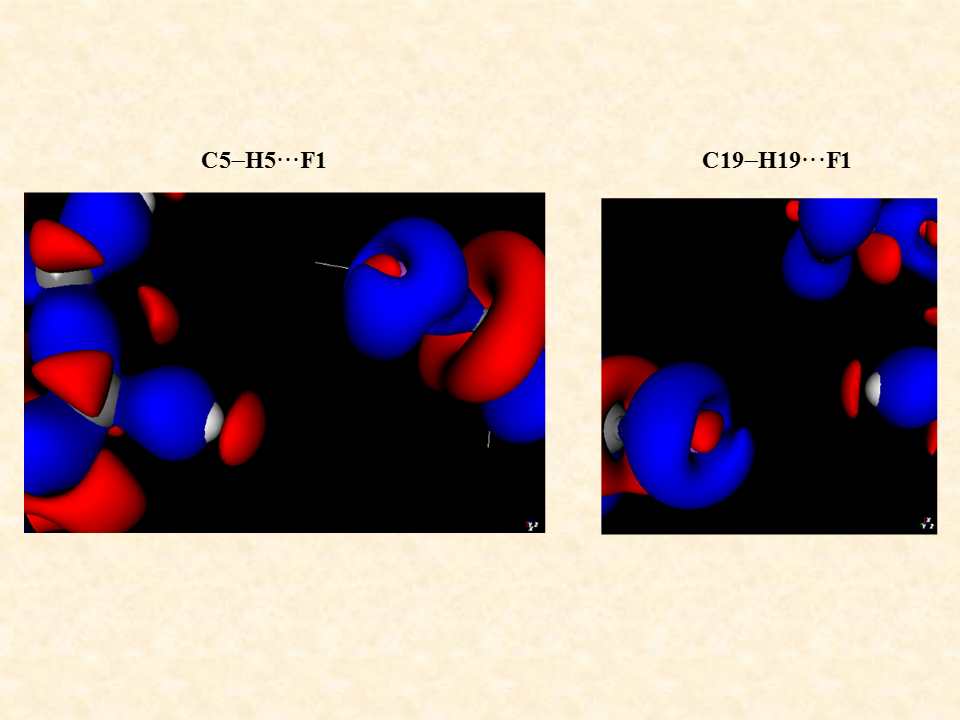
The intermolecular interaction between the molecules is rationalized by the distribution of electron density between the two interacting molecules. The distribution is significantly controlled by the distance and angle between the interacting atoms of the two independent molecules. The standard single crystal X-ray diffraction data enables on to identify the non-hydrogen atoms through their electron density associated with the atoms but the electron density associated with the inter molecular region cannot be ascertained by routine X-ray diffraction data. To visualize the distribution of the electron density of the two interacting molecules near the region of interaction is determined experimentally by recording high resolution X-ray diffraction data generally using X-ray diffractometers equipped with bright Mo or Ag X-ray source. After the structure solution using the standard method one must use the high-resolution data for the multipole refinement of the atoms of the molecule and hence determine the distribution of electron density in the intermolecular region to characterize the weak intermolecular interactions.